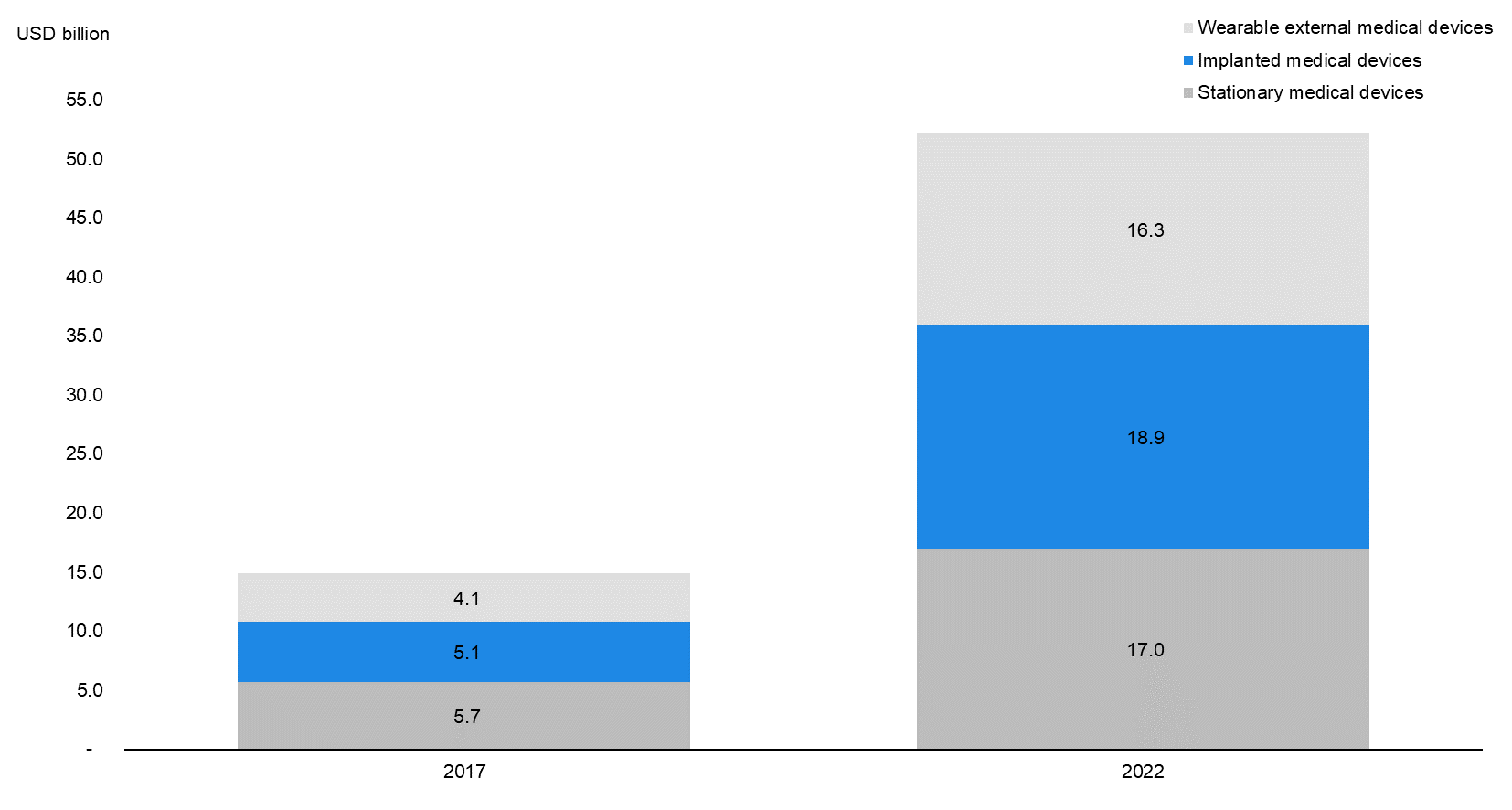Body Talks: The Future of the Connected Implanted Medical Devices Industry
|
The global connected implanted medical devices industry, a key segment of the internet of medical things, is expected to grow at a CAGR of 30.0% over 2017–22E. The high prevalence of cardiovascular diseases and the rising prevalence of neurological conditions are expected to be the main drivers of industry growth. |
|
Moreover, although North America is currently the market’s largest regional segment, the Asia-Pacific region is expected to drive future growth, backed by its developing regional healthcare landscape. Overall, the industry is expected to see continuous investment in product innovation and enhancement, backed by increasing spending on medtech R&D. |
|
The industry is highly concentrated, with the top three players holding a majority share of the market. We believe that these well-established players are expected to maintain their dominant position, considering the high investment, lengthy R&D process, and stringent regulatory requirements the manufacture of these devices involves. Furthermore, growing cybersecurity concerns and the rapid development of the regulatory landscape are expected to be the key challenges in the future. |
|
Connected Implanted Medical Devices a Key Component of the Internet of Medical Things |
|
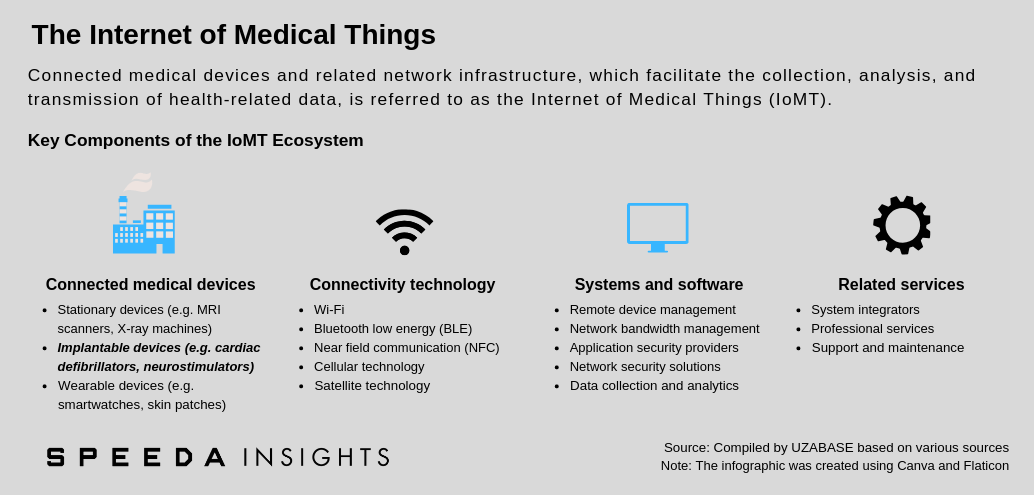
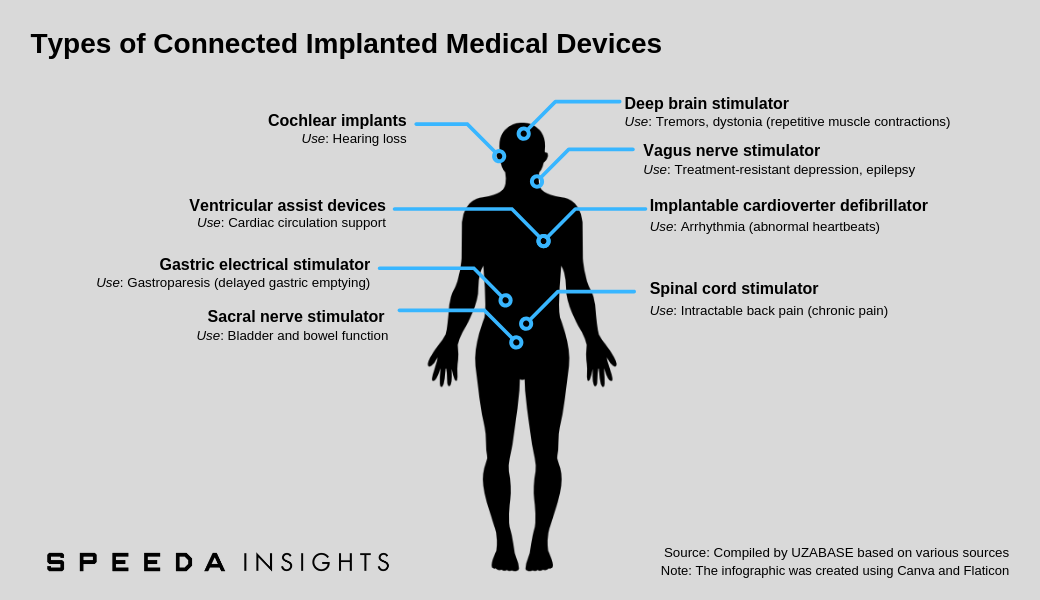
A connected implanted medical device (CIMD; also referred to as an active implanted medical device) is a powered device that is either implanted into a patient’s body following surgical intervention or inserted into a natural orifice. They are generally intended to remain in the patient’s body after the procedure, either for diagnostic purposes or to support a damaged organ or enhance its functionality. These devices may communicate data collected to home transmission units or other connected devices, thereby allowing remote patient follow-up and monitoring. |
|
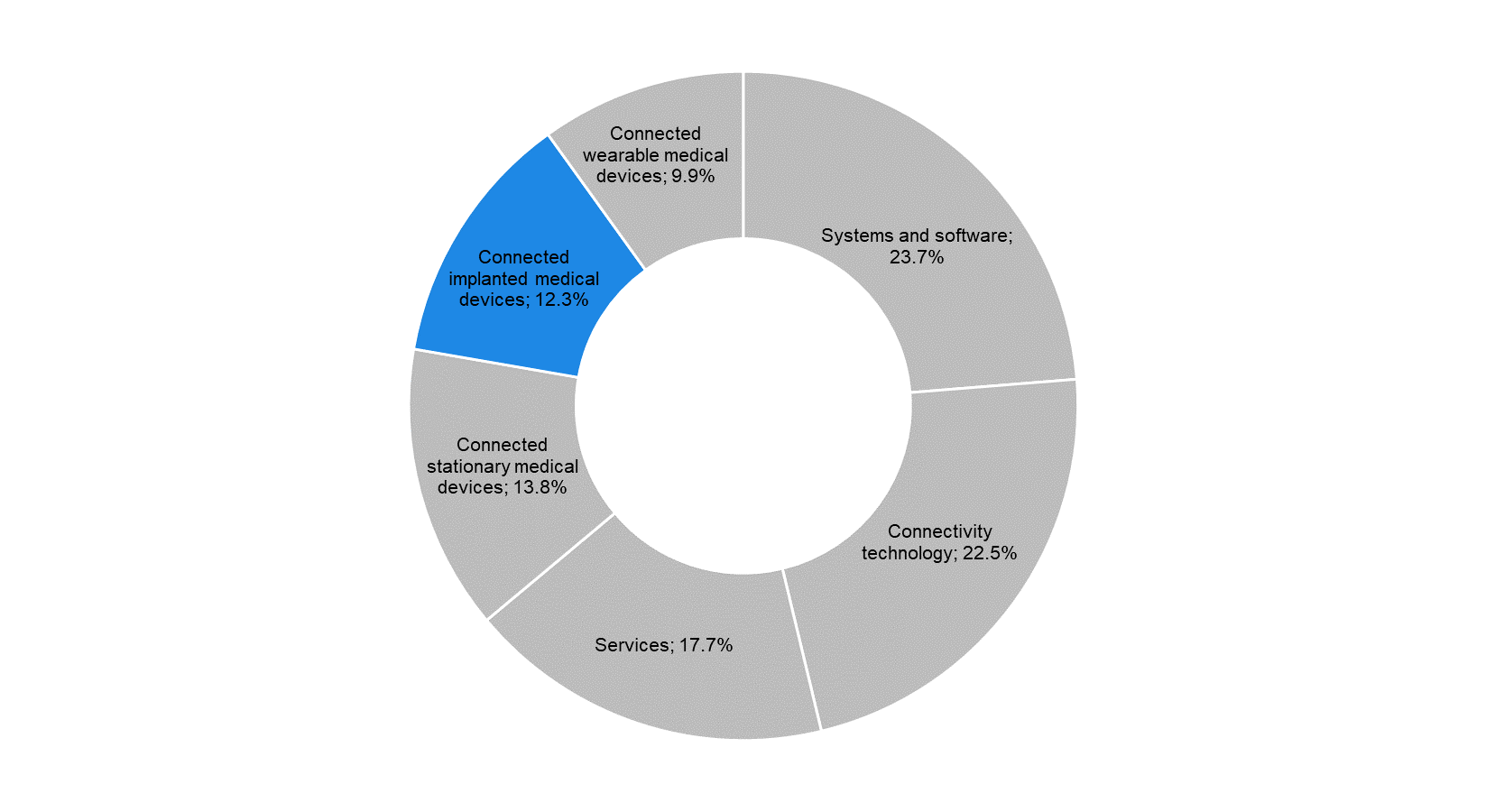
According to data from MarketsandMarkets, the global internet of medical things (IoMT) industry stood at USD 41.0 billion in 2017; of this, CIMDs accounted for 12.3% (USD 5.1 billion). Commonly used CIMDs include cardiac defibrillators and neurostimulators. The IoMT industry also includes stationary devices such as MRI scanners and X-ray machines, as well as wearable devices such as smartwatches and skin patches. These three product segments (implanted, stationary, and wearable devices) are collectively referred to as ‘connected medtech devices’ and formed the largest share (36.1%) of the total IoMT market in 2017. |
|
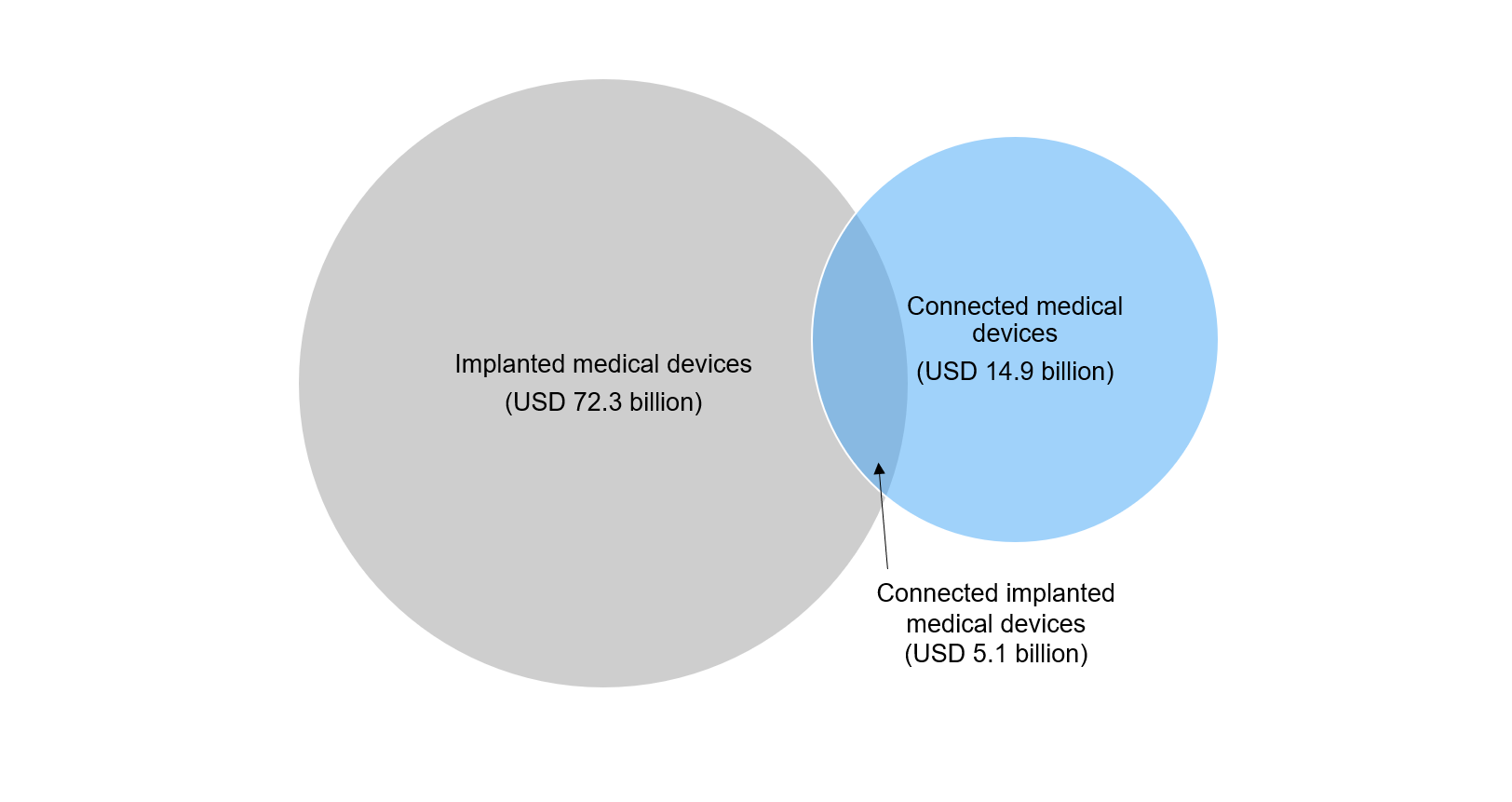
CIMDs are also related to the broader implanted medical devices industry, which comprises inactive medical devices such as orthopaedic implants, hip implants, and breast implants. Implanted medical devices is a relatively mature industry; its market size was around USD 72.3 billion in 2017, as per estimates by Allied Market Research. |
|
CIMDs Account for Around One-Eighth of Global IoMT Industry, 2017 |
|
|
|
Source: MarketsandMarkets research based on Deloitte’s “Medtech and the Internet of Medical Things” |
|
CIMDs Form Part of the Overall Implanted Medical Devices Industry, 2017 |
|
|
|
Source: MarketsandMarkets, Allied Market Research |
|
|
|
|
|
Implanted Medical Devices to Become the Largest Segment in Connected Medtech by 2022E |
|
According to data by MarketsandMarkets, the CIMD segment is expected to grow at a CAGR of 30.0% to USD 18.9 billion in 2022E from USD 5.1 billion in 2017. CIMDs are expected to account for 36.2% of the overall connected medtech market in 2022E (34.2% in 2017), overtaking stationary medical devices (32.6% in 2022E) as the largest product segment. |
|
Implanted Medical Devices to Dominate Connected Devices Market by 2022E |
|
|
|
Source: MarketsandMarkets research based on Deloitte’s “Medtech and the Internet of Medical Things” |
|
Note: Data for 2022 is an estimate |
|
The principal factors supporting this strong growth in the CIMD market are identified below. |
|
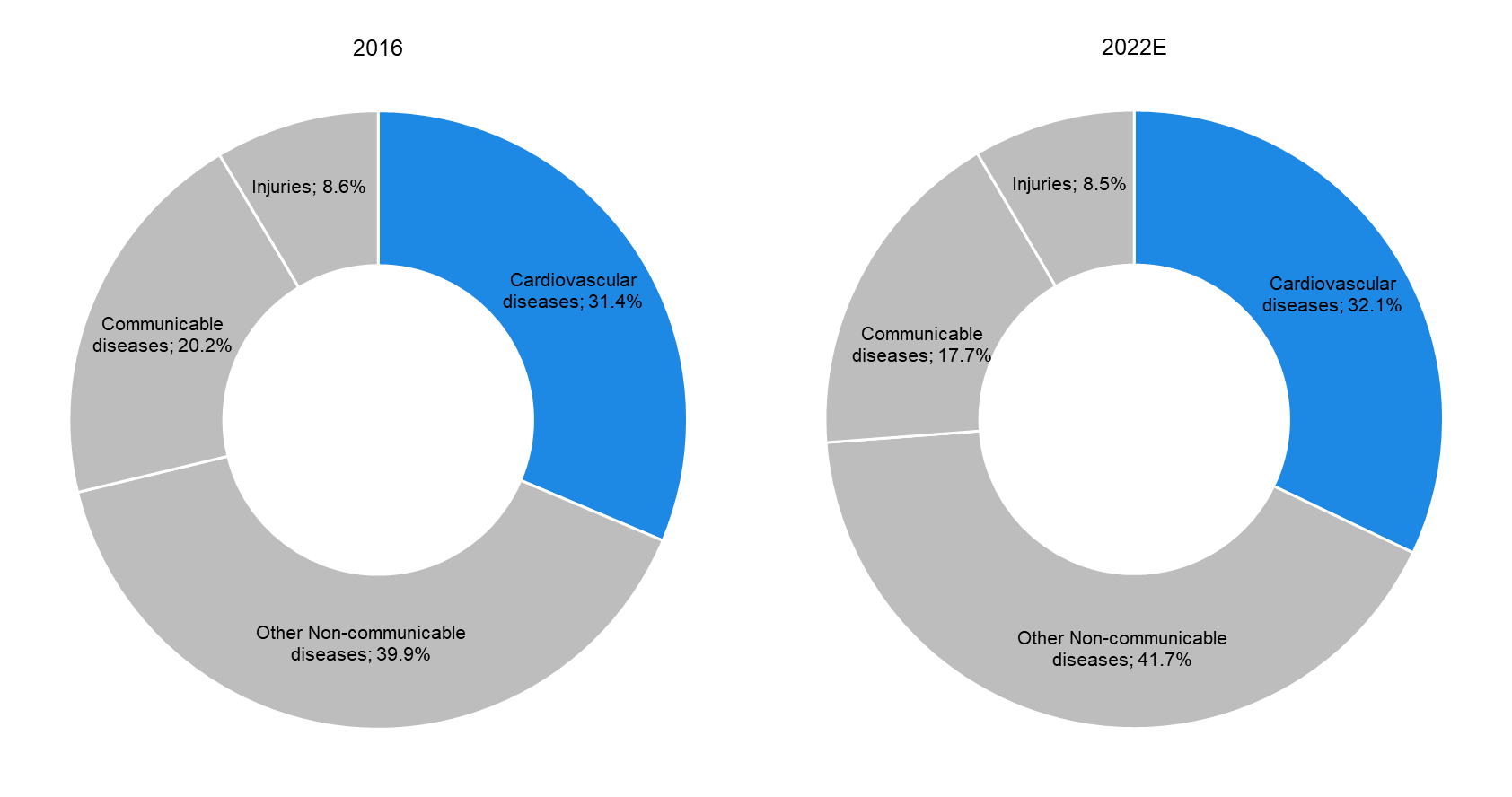
1. High Prevalence of Cardiovascular Diseases to Drive Demand for Implanted Cardiovascular Products |
|
According to data from the WHO, cardiovascular diseases (CVDs) are expected to remain the leading cause of death globally, accounting for almost one-third (32.1%) of total global deaths in 2022E (31.4% deaths in 2016). This is attributable to the high prevalence of avoidable risk factors such as unhealthy diet, physical inactivity, and tobacco and alcohol use. CVDs are most common in the elderly (with around 83% of global CVD-related deaths occurring in people above 60 years of age); this offers significant growth opportunities in the market for advanced implanted cardiovascular products. |
|
As per MarketsandMarkets, implantable cardioverter defibrillators accounted for the largest share of the implanted medical devices industry in 2016 (latest data available). Implantable cardioverter defibrillators can continuously monitor patient heart rhythm and send electrical impulses if any irregularities in rhythm are detected. They are used mainly to prevent sudden cardiac arrest in high-risk patients, including those who have survived a sudden cardiac arrest, those who developed arrhythmia after a heart attack and those who are genetically predisposed to arrhythmia (e.g. due to congenital heart disease). |
|
Going forward, growth opportunities in product design lie in the development of miniaturised implants that require minimally invasive procedures and could eliminate complications related to infection during surgery and also shorten hospital stays. Advanced devices with longer battery life or rechargeable capabilities are also areas of development. |
|
Cardiovascular Diseases to Remain the Leading Cause of Death Globally, 2016–22E |
|
|
|
Source: WHO |
|
Note: Data for 2022 is an estimate |
|
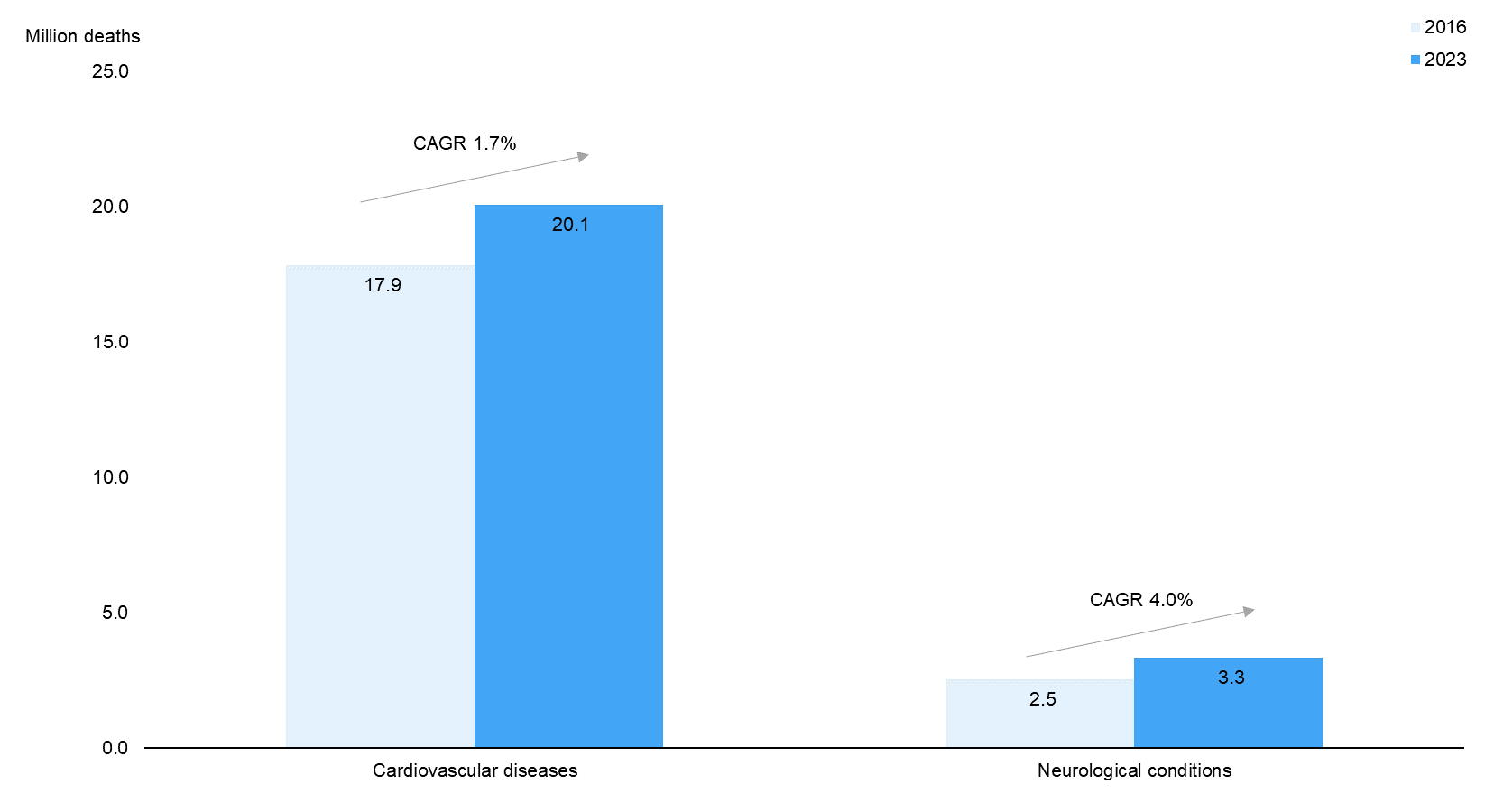
2. Rapidly Rising Prevalence of Neurological Diseases to Fuel Demand for Implanted Neurostimulation Devices |
|
As per P&S Market Research, the market for neurostimulation devices is expected to grow considerably over 2016–23E. Demand for innovative neurostimulation devices is backed by the rising global prevalence of neurological conditions such as Alzheimer’s, Parkinson’s, epilepsy, and multiple sclerosis, which have no known cure. According to data from the WHO, the number of deaths due to neurological conditions (4.5% of total deaths in 2016) is expected to increase at a rapid CAGR of 4.0% over 2016–23E, notably faster than the increase in deaths due to cardiovascular diseases (CAGR of 1.7%) and overall deaths due to non-communicable diseases (NCDs; CAGR of 1.9%). |
|
Neurological conditions tend to be progressive, with patients suffering from related symptoms, such as chronic pain, urinary and faecal incontinence, depression, dystonia, and tremors, for extensive periods. Moreover, such conditions generally require multiple, lengthy hospitalisations. Neurostimulation devices could help in early diagnosis and control of related symptoms and potentially help improve the quality of life of patients, especially of those that no longer respond to medications. |
|
Moreover, in recent years, neurostimulation devices have been receiving regulatory approval for an extensive range of applications (the US FDA first approved the use of a deep brain stimulator in 1997), including Parkinson’s, epilepsy, and obsessive-compulsive disorder (OCD). This indicates the increasing success rate of trials and a favourable opinion on the use of such devices, despite prevailing concerns about the high cost of neurosurgery and the limited network of skilled healthcare professionals capable of conducting such procedures. |
|
Deaths due to Neurological Conditions to Increase Significantly over 2016–23E |
|
|
|
Source: Created by UZABASE based on data from the WHO |
|
Note: Data for 2022 is an estimate |
|
3. Improved Affordability and Developing Health Infrastructure to Drive Growth in Currently Underpenetrated Asia-Pacific Region |
|
According to MarketsandMarkets, North America accounted for the largest share of the global implanted medical devices industry’s revenue in 2016 (latest data available). This was mainly due to the high accessibility of innovative and technologically advanced medical devices, backed by the presence of well-established healthcare systems and favourable reimbursement policies. However, the Asia-Pacific region is expected to be the key driver of future growth. This should be influenced mainly by the rapid development of healthcare infrastructure in emerging markets such as China and India (which collectively account for over one-third of the global population in 2017). In fact, healthcare expenditure per capita grew at a higher rate in China (CAGR of 13.4% over 2010–15, latest data available) and India (CAGR of 10.3%) than in leading healthcare spenders such as the USA (CAGR of 3.7%) and Switzerland (CAGR of 5.8%). Developments in healthcare infrastructure, backed by increasing demand for advanced medtech devices, should further improve reimbursement policies, making advanced implanted devices accessible and affordable to a wider population. |
|
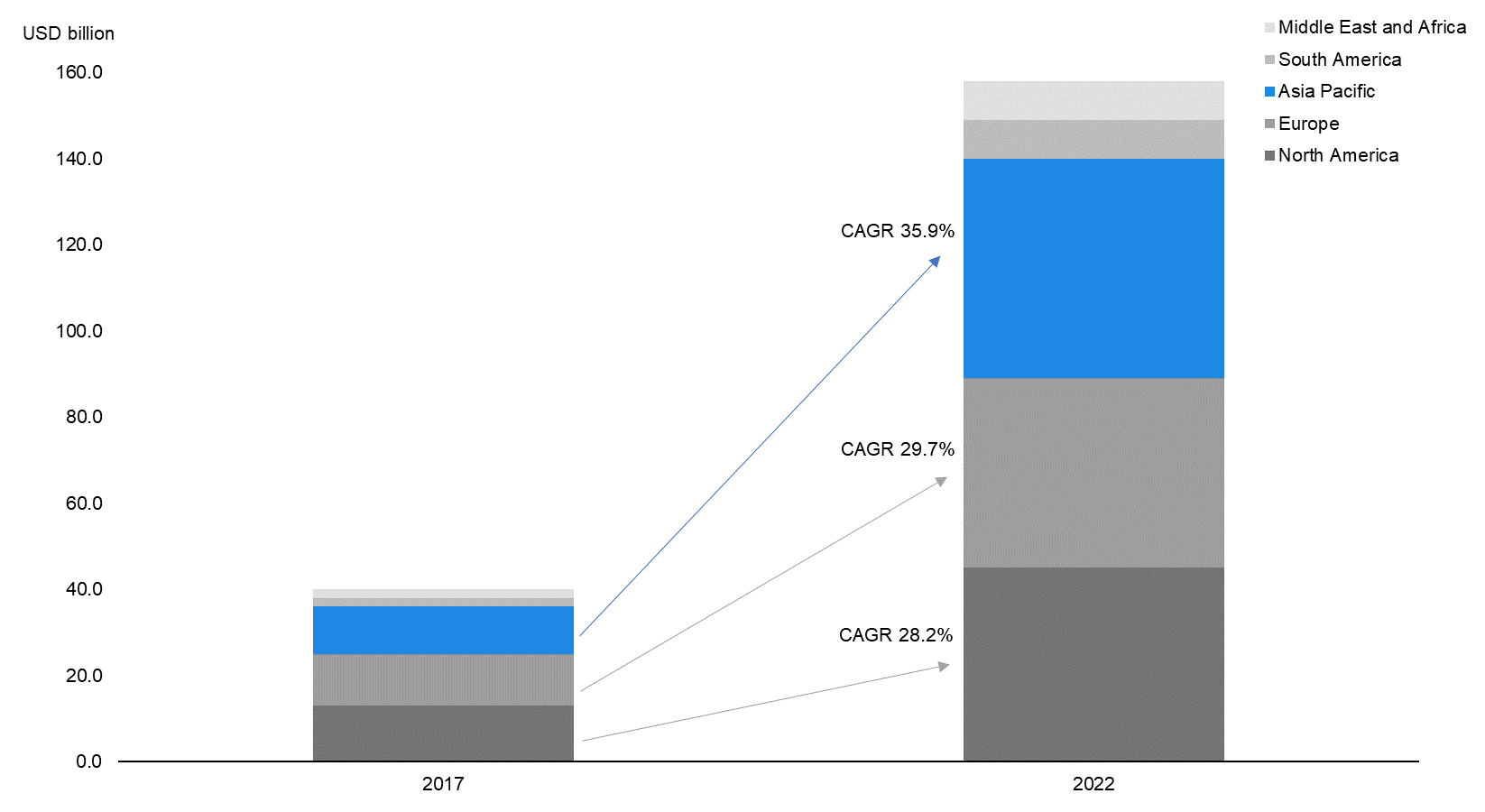
The regional growth trends above are also reflected in the overall IoMT market. As per data by MarketsandMarkets, IoMT market revenue in the Asia-Pacific region is expected to grow at a CAGR of 35.9% over 2017–22E, faster than in North America (CAGR of 28.2%) and Europe (CAGR of 29.7%). |
|
Asia Pacific to Dominate Overall IoMT Market by 2022E |
|
|
|
Source: MarketsandMarkets research based on Deloitte’s “Medtech and the Internet of Medical Things” |
|
Note: Data for 2022 is an estimate |
|
4. Continuous Product Innovation and Enhancement, Backed by Investment in Medtech R&D, to Support Overall Industry Growth |
|
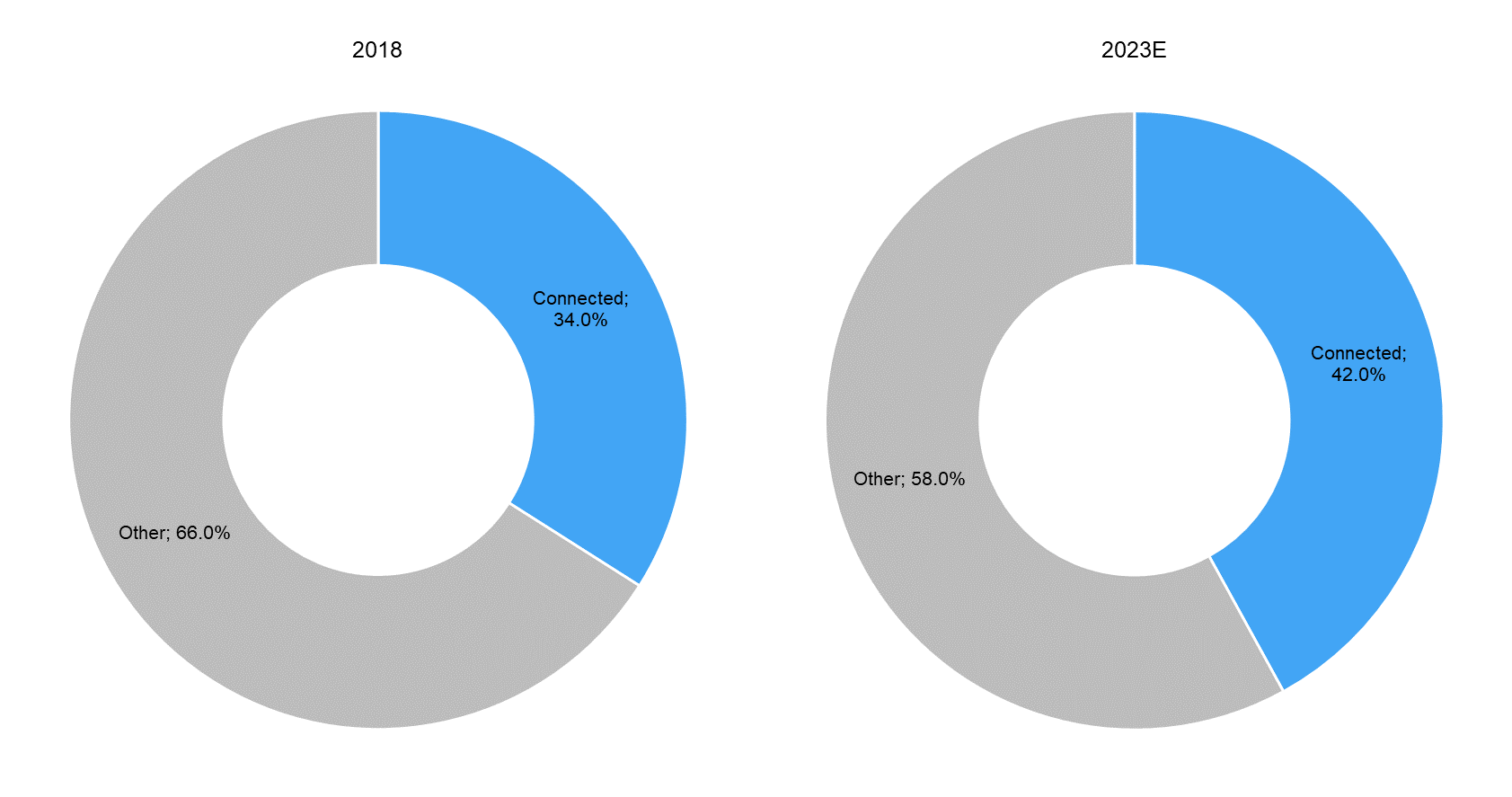
According to a report by Deloitte, connected medtech devices are generally required to be upgraded or replaced every 18–24 months. Thus, continuous product innovation and enhancement is a key feature of the overall connected medtech industry, backed by the expected increase in the number of patients who are remotely monitored through connected devices (to over 50 million in 2021E from around 7.1 million in 2016, as per Berg Insight) and the overall increase in investment in medtech R&D by manufacturers. According to a survey conducted by Deloitte in 2018 on connected medtech devices, R&D budget allocation for the development of connected medtech devices is expected to increase to around 42% of the total R&D budget of medtech companies within the next five years, from around 34% in 2018. |
|
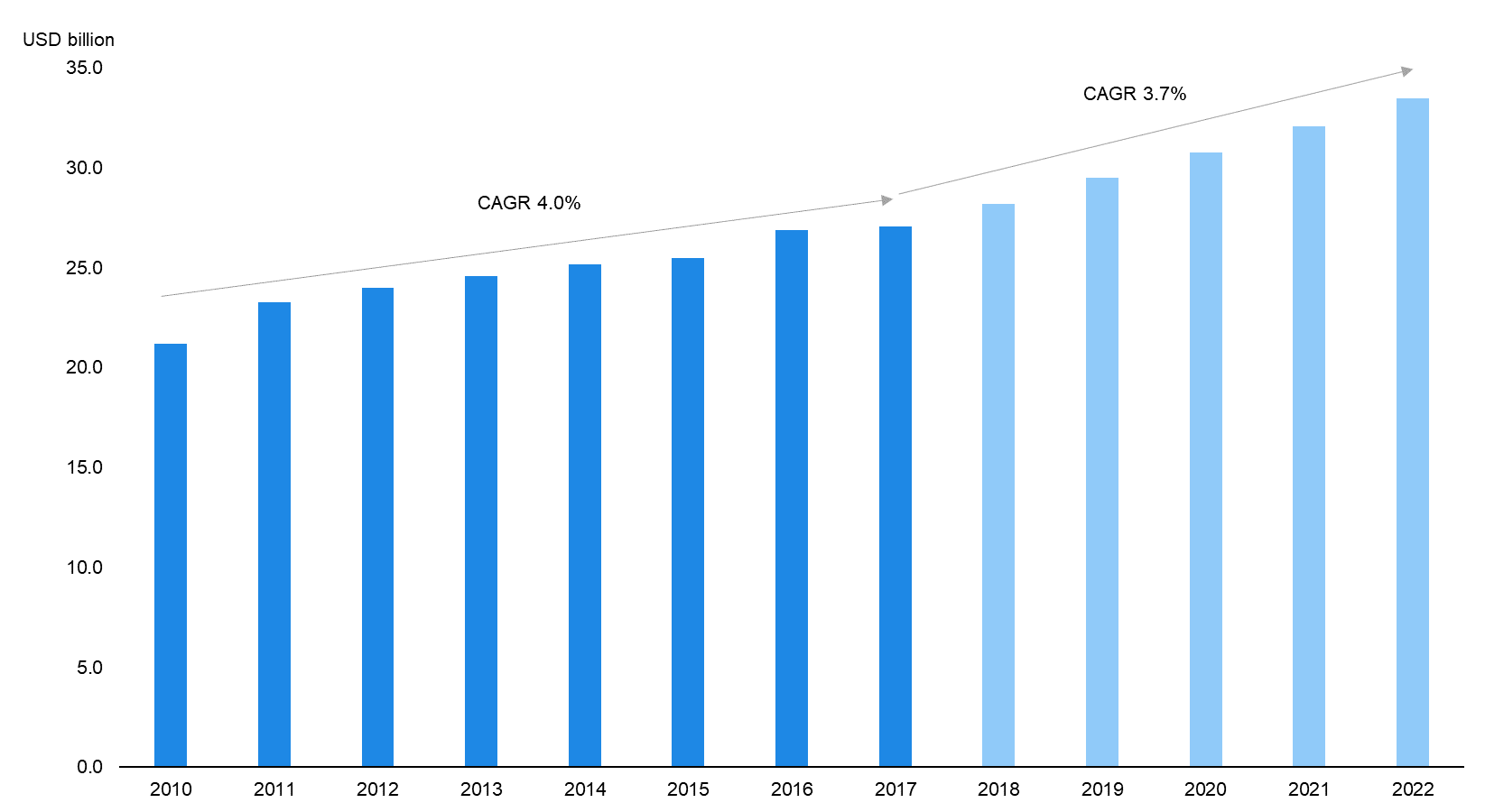
In addition, as per Evaluate Pharma, investments in global medtech R&D is expected to grow steadily at a CAGR of 3.7% to USD 33.5 billion in FY2022E (6.4% of global medtech sales) from USD 26.9 billion (7.0% of global medtech sales) in FY2016 (latest data available). This should be backed by rising global spending on healthcare, which is expected to grow at a CAGR of 4.3% to USD 8,734 billion in 2020E from USD 7,077 billion in 2015 (latest data available), according to a report by Deloitte. The high prevalence of chronic conditions (accounting for 71.3% of global deaths in 2016, latest data available as per the WHO) and global population ageing (people aged 60 years and above should more than double to around 2,100 million in 2050E from about 962 million in 2017, as per the UN) are also expected to be key factors driving the market. |
|
R&D Budget Allocation for Development of Connected Medtech Devices to Increase over 2018–23E |
|
|
|
Source: Deloitte, “Medtech and the Internet of Medical Things” |
|
Note 1: Survey data based on the responses of 237 companies |
|
Note 2: Data for 2022 is an estimate |
|
Global Medtech R&D Spend to Grow Steadily over FY2016–22E |
|
|
|
Source: EvaluatePharma, “World Preview 2017, Outlook to 2022” |
|
Note: Data from 2018 onwards comprises estimates |
|
Industry to Remain Highly Concentrated, with Top Three Players Expected to Maintain Dominant Positions |
|
The global CIMD market remains highly consolidated. The top three players, Medtronic (USA), Abbott Laboratories (USA), and Boston Scientific (USA), collectively accounted for around 85% of the implanted cardiovascular devices segment in 2015 (latest data available) and around 95% of the implanted neurostimulator devices market, as per MarketsandMarkets. |
|
According to a report by AARP (USA), CIMDs generally involve a relatively lengthy R&D process and require significant investment, and this limits market competition. Stringent regulatory requirements, in terms of a complex approval process and patent protection schemes, is a contributing factor. A lack of price transparency for CIMDs also limits competition, as it allows manufacturers the opportunity to charge relatively high prices for products. The existing established players are therefore likely to benefit the most from the projected growth in the industry. |
|
Nevertheless, numerous start-ups that focus on the development of novel implanted devices are also present in the market, given the industry’s growth prospects. For instance, Cardialen (USA) is developing a low-energy implantable defibrillator, while Axonics (USA) and Synergia Medical (BEL) are working on implantable neuromodulation technology. |
|
|
|
Cybersecurity Concerns and Increasing Implementation of Medtech Regulations to be the Main Challenges for Players |
|
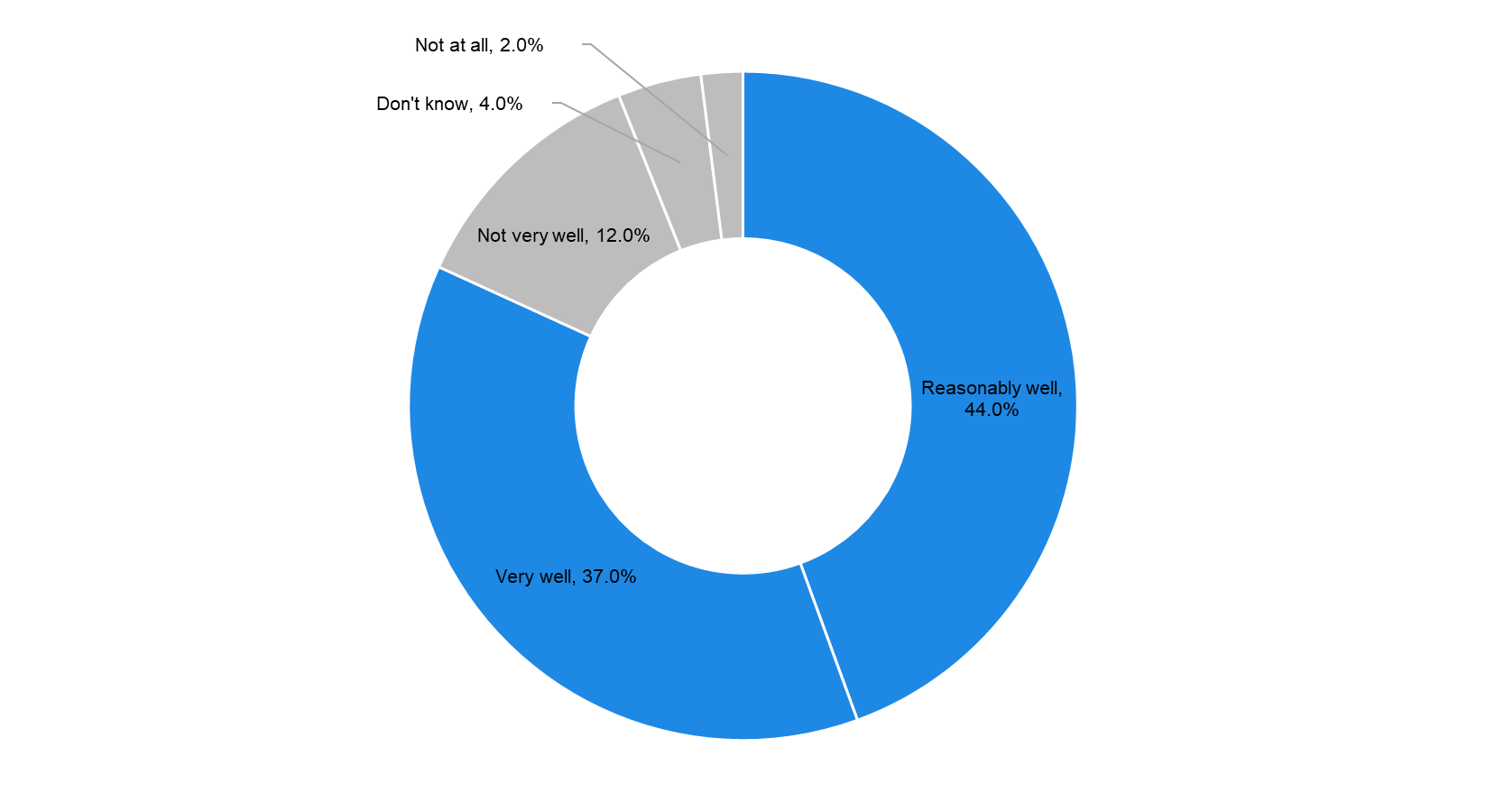
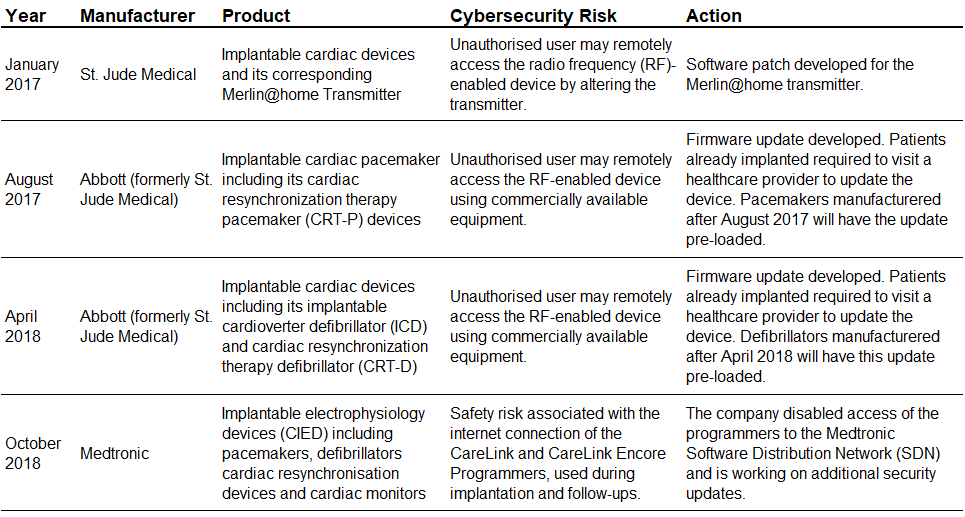
Despite a lack of reports of actual harm to patients, concern about the cybersecurity vulnerabilities of implanted devices is growing. A hacker could change the functionality of an implanted device, and this poses a major threat to patient safety, especially for high profile individuals. For instance, former US Vice President (2001–09) Dick Cheney stated in his book (Heart: An American Medical Odyssey, 2013) that he had ordered himself an implanted cardiac defibrillator without wireless capabilities, due to the fear of an assassination by an unwarranted electric shock to his cardiac device. However, in a survey conducted by Deloitte in 2018 on connected medtech devices, the majority of respondents stated that they perceived their organisation to be ‘reasonably well’ or ‘very well’ prepared to maintain the cybersecurity of their medtech devices. |
|
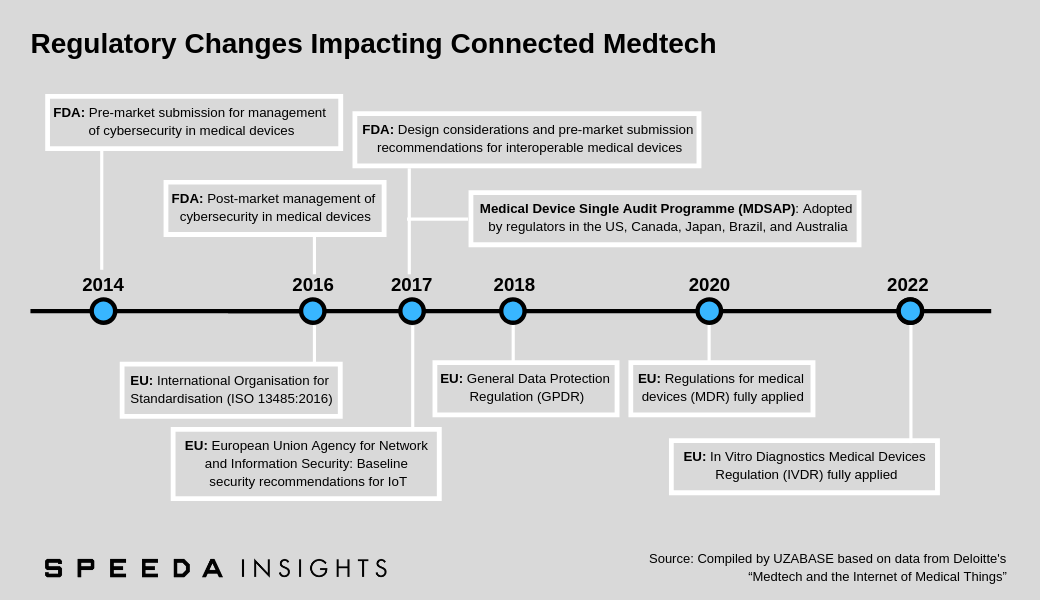
Nevertheless, given the growth of the IoMT landscape, regulatory bodies in leading countries have taken steps to implement legislation to manage cybersecurity and the overall safety of connected medtech devices. The US FDA, for instance, issued pre-market (2014) and post-market (2016) guidance submissions for the management of cybersecurity in medical devices. Moreover, since 2017, the FDA has also reviewed multiple product-specific cybersecurity vulnerabilities in CIMDs (the first cybersecurity safety alert was issued in May 2015). This has led product manufacturers to develop software and firmware updates to prevent unauthorised access to devices. Going forward, a key regulatory change is expected in Europe, with the EU Medical Device Regulation set to come into force in 2020. |
|
Therefore, ensuring that cybersecurity vulnerabilities are addressed in the original product design itself and navigating through the rapidly developing regulatory landscape should be the key challenges to overcome in the future. |
|
Majority of Medtech Companies Seem Well Prepared to Maintain Cybersecurity, 2018 |
|
|
|
Source: Deloitte, “Medtech and the Internet of Medical Things” |
|
Note: Survey data based on the responses of 237 companies |
|
FDA Cybersecurity Safety Communications Related to Implanted Devices |
|
|
|
Source: Compiled by UZABASE based on data from the US FDA |
|
|